Apple Inc received FDA’s 510(k) clearance for a novel AFib feature that’s available on Apple Watch using watchOS 9 and above.
Your Apple Watch can now keep track of an adult’s (22 years and older) Atrial Fibrillation history once diagnosed with AFib by a doctor or healthcare provider. 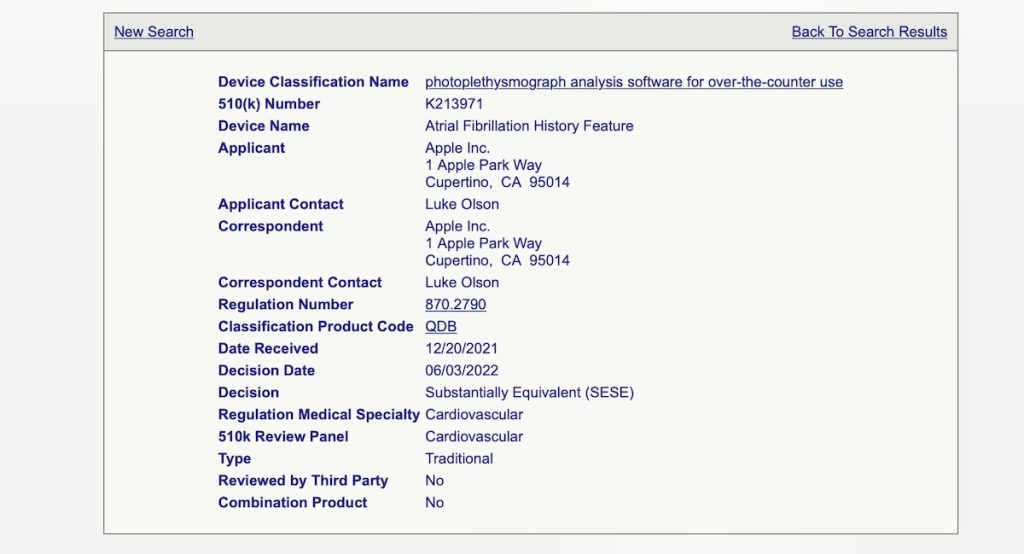
Users in the US aged 22 years or older already diagnosed with AFib can turn this feature on in the Health app and then should be able to check their AFib history and more–a first for any wearable!
For older model Apple Watches that don’t support watchOS 9+, Apple continues to recommend that people use the ECG app and irregular rhythm notifications on the Apple Watch to identify potential signs of atrial fibrillation (AFib.)
Contents
Related reading
- How to use the Irregular Heart Rhythm notification feature on Apple Watch
- Apple Watch able to detect arrhythmias other than AFib ( new findings from Apple Heart Study)
- How to set up and use an Apple Watch for parents or an older adult in your care
How to show your AFib History on Apple Watch and the Health app
Using watchOS 9 and above, Apple Watch users diagnosed with atrial fibrillation can use AFib History to monitor their condition.
Atrial fibrillation (AFib) is a chronic condition where an irregular heart rhythm is caused when the upper chambers of the heart beat out of sync with the lower chambers.
AFib and other irregularities in heart rhythm are more common as people age. While some people with AFib don’t experience any symptoms, many experience symptoms such as rapid heartbeat, palpitations, fatigue, or shortness of breath.
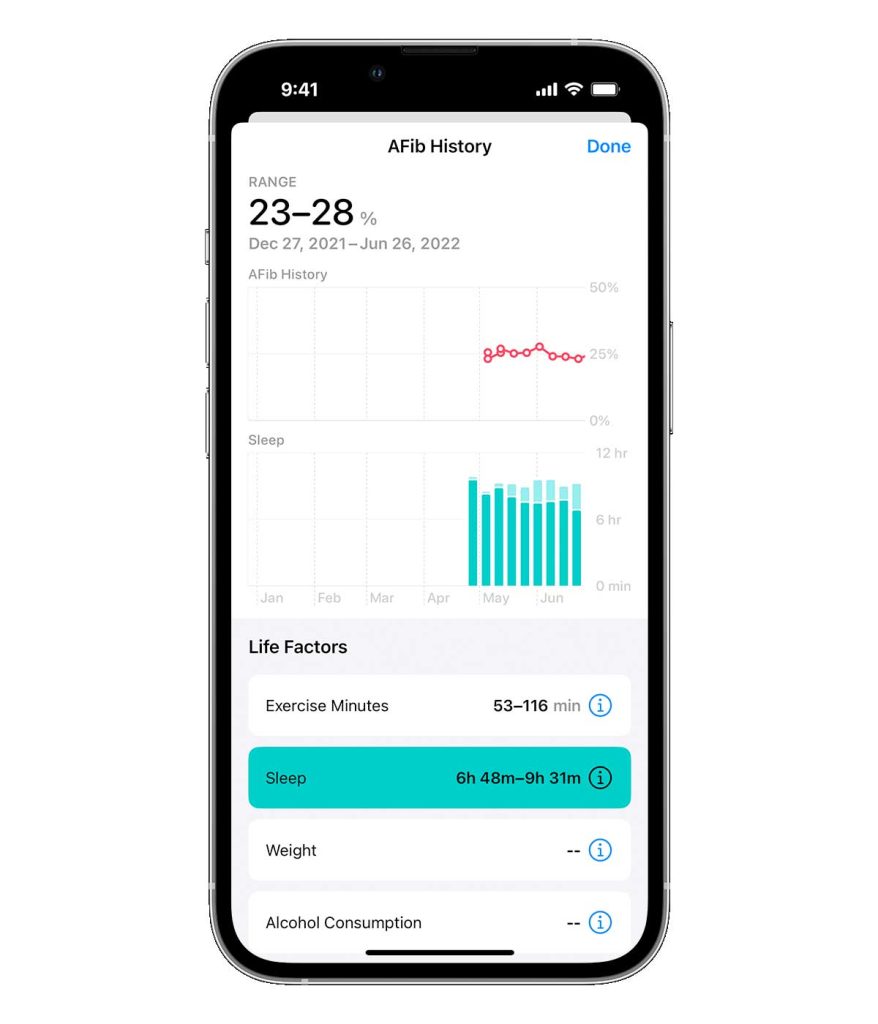
Apple’s AFib history feature estimates the time a user’s heart is in AFib (called AFib burden) and helps users stay informed about lifestyle factors that may influence AFib. You can also share your AFib history with your healthcare team.
You should only set this feature up if you have been diagnosed with AFib. Also, this feature is not available in all regions, so you may not be able to set it up.
Additionally, you must meet the following:
- Update your iPhone and Apple Watch to the latest version of iOS and watchOS–and at least watchOS 9.
- Do not use Low Power mode on your watch since it turns off background measurements.
- Wear your Apple Watch at least 12 hours a day for 5 days a week to consistently receive estimates.
- Turn on Heart Rate and Wrist Detection on your Apple Watch.
- At least 22 years old.
- You cannot have both Irregular Heart Rhythm Notifications and AFib History both on. Irregular rhythm notifications are turned off when you set up AFib History.
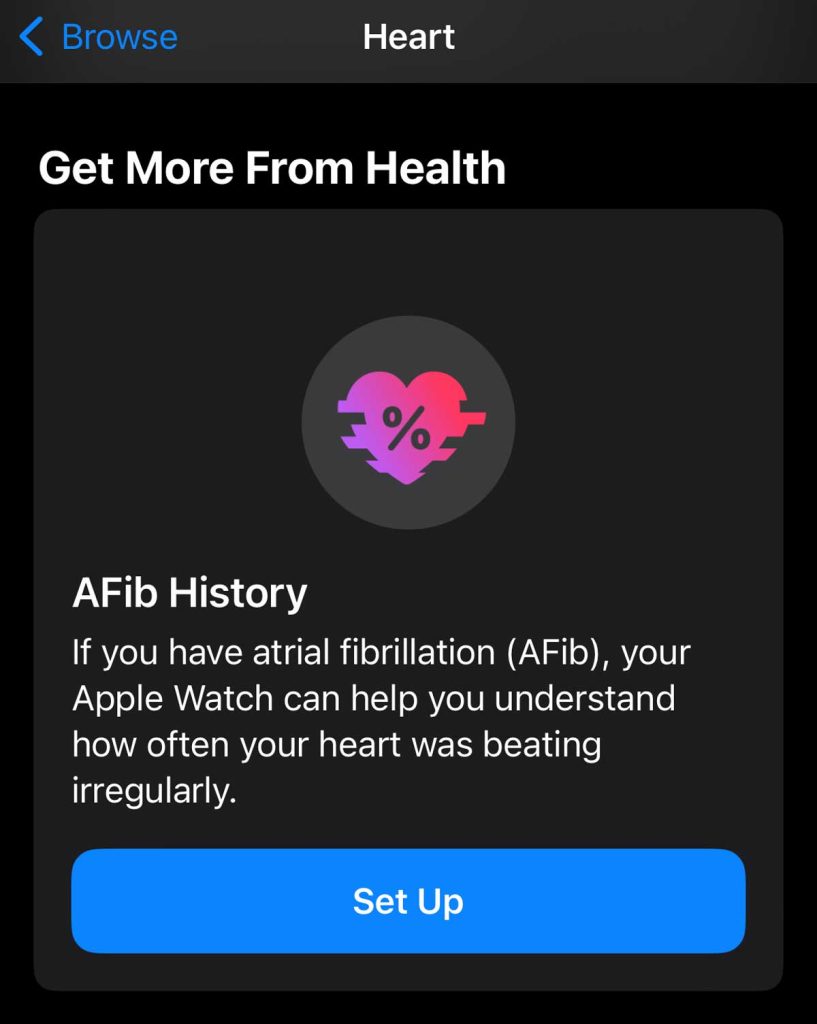
Set up AFib History tracking
- Open the Health app on your iPhone, then tap Browse.
- Choose the Heart category
- Scroll down to the section Get More From Your Health and in the AFib History tile, tap Set Up.

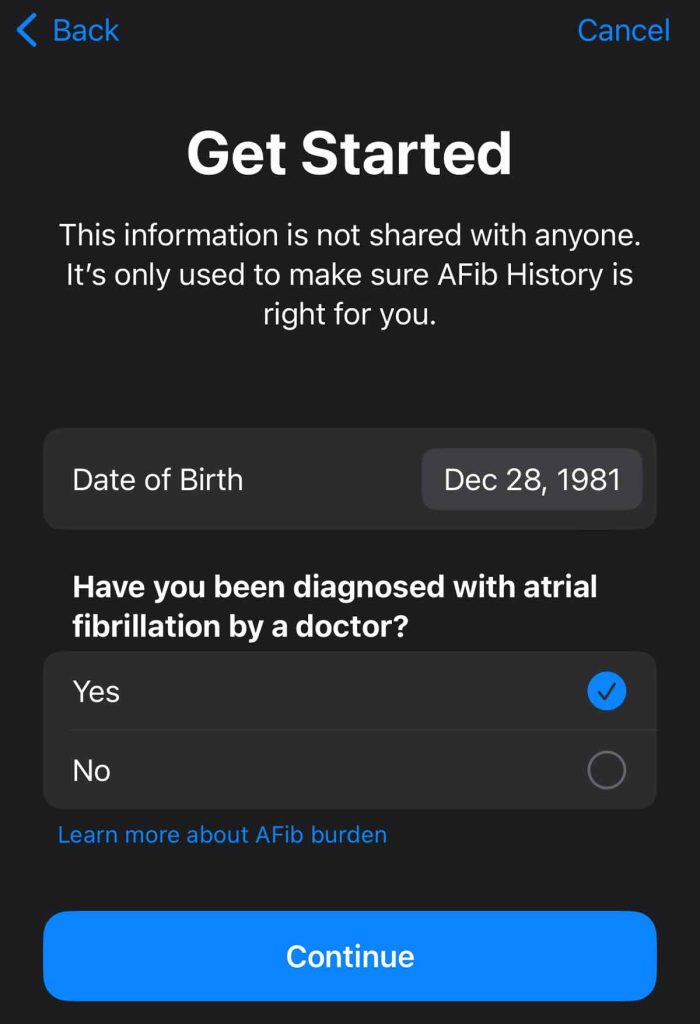
- Tap Get Started.
- AFib history uses your location to determine if this feature is available in your country or region.

- AFib history uses your location to determine if this feature is available in your country or region.
- Enter your Date of Birth (you must be at least 22 years old.)
- Confirm you have been diagnosed with AFib by a doctor by tapping Yes and then tapping Continue.

- Tap Continue to learn more about AFib History, the results you may see, and life factors.
- Tap Done.
When you turn on this feature, your Apple Watch only checks for signs of atrial fibrillation periodically.
AFib History may not find every instance of your irregular rhythm. Also, AFib History does not notify you when you’re experiencing AFib in real time.
Review your AFib History 
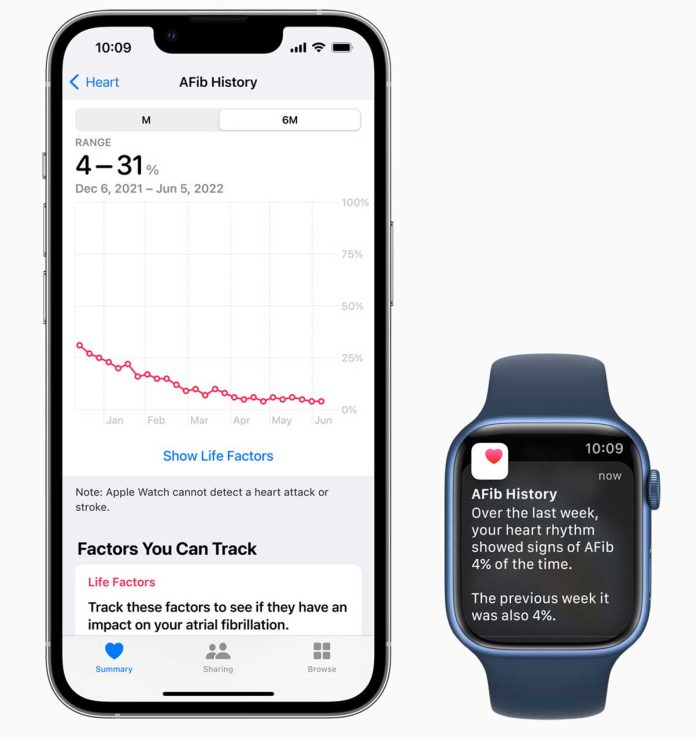
To review your AFib history, open the Health app, tap Browse, tap Heart, then tap AFib History. Your AFib History estimate appears as a percentage.
- A lower percentage means your heart was in AFib less often.
- Your AFib History never shows as 0%. Instead, it may show as 2% or less.
- A higher percentage means your heart was in AFib more often.
- Tap Show Life Factors to compare a Life Factor, like sleep or exercise, with your AFib History.
Every Monday, if you have worn your watch for at least 5 of 7 days (12 hours a day), you may receive a notification with an estimate of the time you spent in AFib during the previous calendar week.
An AFib result means the heart is beating in an irregular pattern. The ECG app ver. 1 can check for AFib between 50 and 120 BPM. The ECG app ver. 2 can check for AFib between 50 and 150 BPM.
AFib is the most common form of serious arrhythmia or irregular heart rhythm. If you receive an AFib classification and have not been diagnosed with AFib, you should talk to your doctor.
Send your AFib history to a healthcare provider or a caregiver
To export your AFib History data as a PDF, scroll down to Options and tap Export PDF. Tap the Share Button to send the PDF to someone in your Contacts, save it to the Files app, or AirDrop to another device.
You can also share your AFib History and other health data in the Health app by choosing to share with someone or share with your doctor in the Sharing tab at the bottom of the Health app.
Wrap up
According to Apple, the ability of the ECG app to accurately classify an ECG recording into AFib and sinus rhythm was tested in a clinical trial of approximately 600 subjects. It demonstrated 99.6% specificity with respect to sinus rhythm classification and 98.3% sensitivity for AFib classification for the classifiable results.
Apple’s press release for watchOS 9 described this new health feature as doing the following:
With watchOS 9, users who are diagnosed with AFib can turn on the FDA-cleared AFib History feature and access important information, including an estimate of how frequently a user’s heart rhythm shows signs of AFib, providing deeper insights into their condition. Users will also receive weekly notifications to understand frequency and view a detailed history in the Health app, including lifestyle factors that may influence AFib, like sleep, alcohol consumption, and exercise.Users can download a PDF with a detailed history of their AFib and lifestyle factors, which can easily be shared with doctors and care providers for more informed conversations.
This feature is available on Apple Watches that support watchOS 9 and above.








I like this app
The 510(k) process is not considered an “approval”. Devices are “cleared” through the 510(k) process.
“Approvals” are limited to the higher risk devices and indicates a much higher level of review by the FDA through the Premarket Approval (PMA) process.